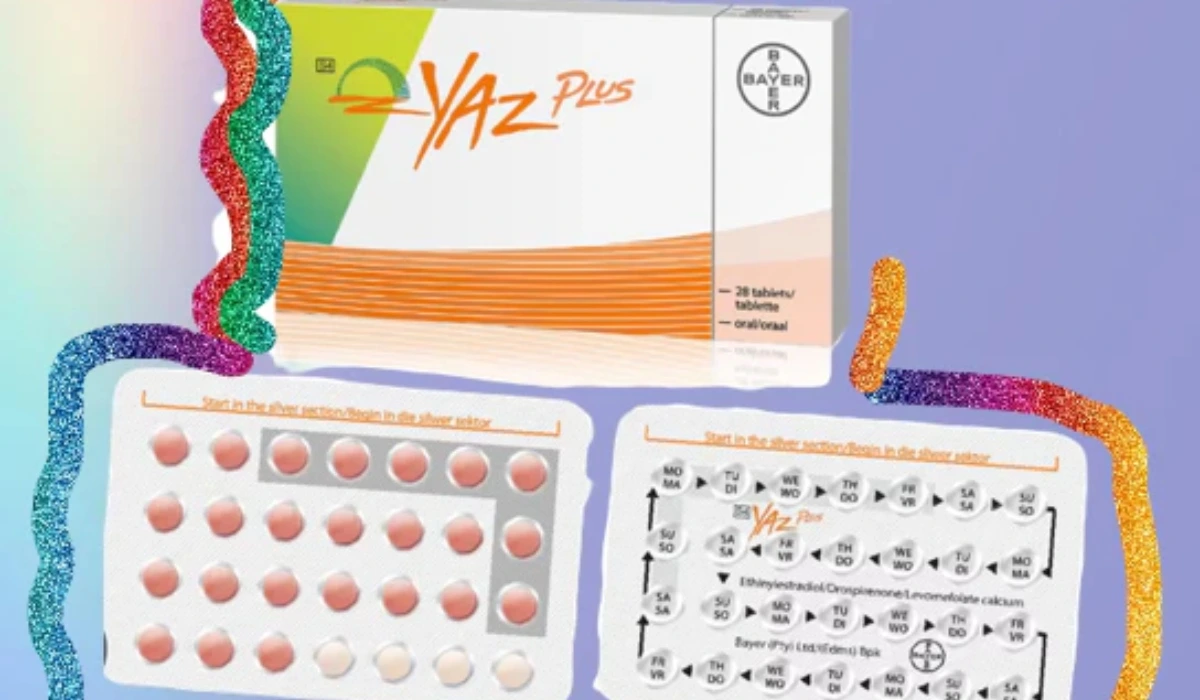
Regulators in South Africa have urgently recalled a batch of Yaz Plus contraceptive pills after a packaging error rendered the pills potentially ineffective, raising concerns about unintended pregnancies.
Popular Contraceptive Pill Yaz Plus Recalled
The recall involves batch WEW96J, which expires in March 2026 and was prompted by a manufacturing mix-up where some blister packs contained 24 inactive hormone-free pills instead of the intended 24 active, hormone-containing pills.
Manufacturer Bayer Ltd has advised women using the affected batch to stop immediately and consult their healthcare providers.
“While only a limited number of packs from the respective batch is affected, as a precautionary measure, no tablets from these packs shall be used until you have consulted your healthcare practitioner, as they may potentially not provide the contraceptive protection you expect,” the company said.
The recall, conducted in collaboration with the South African Health Products Regulatory Authority (SAHPRA), applies to consumers, pharmacies, and healthcare facilities. Individuals who purchased the affected batch are encouraged to return the product for a replacement or refund.
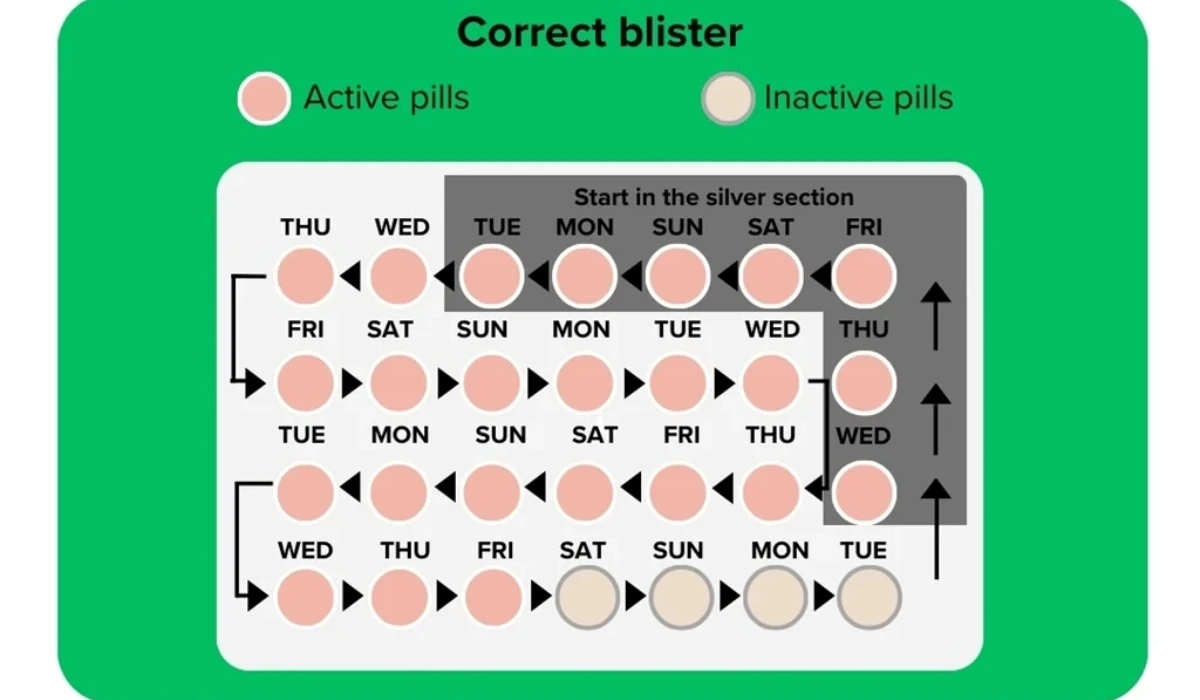
The mix-up has been traced to packaging errors, with corrective actions already implemented. In the flawed packs, 24 hormone-free orange pills were mistakenly packaged as active pills, leaving only four hormone-containing pink pills in each pack. Normally, a Yaz Plus pack contains 24 active pink pills followed by four inactive orange pills.
Bayer assured the public that no other batches were affected and stressed the issue was isolated.
“The root cause for the mix-up of tablets in the packaging has been identified, and corrective measures have been implemented,” the company stated.
The company has also set up a helpline to address concerns from affected consumers and healthcare professionals.
SAHPRA Confirms The Error
SAHPRA said in a statement:
“The South African Health Products Regulatory Authority (SAHPRA) is aware of a quality issue in a batch of YAZ Plus contraceptives (Batch No. WEW96J distributed on 09 and 24 November 2023), which has resulted in the incorrect arrangement of tablets within some packs, thus affecting the efficacy of the affected batch.
“Instead of containing 24 pink film-coated hormone tablets and 4 light orange hormone-free tablets, the affected packs contain 24 light orange hormone-free tablets and 4 pink film-coated hormone tablets. SAHPRA is collaborating with the manufacturer, Bayer, to ensure a speedy recall.“